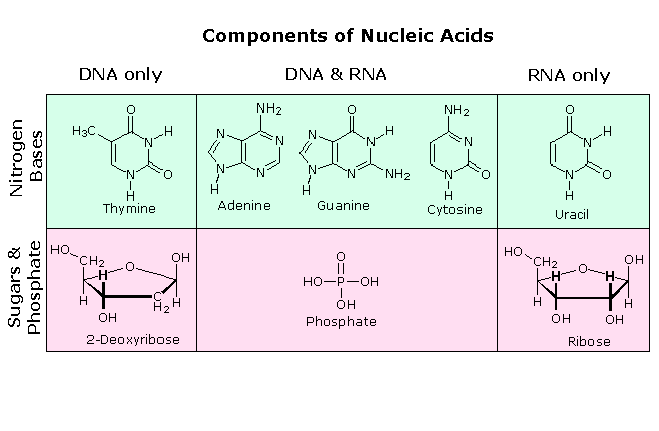
In RNA, uracil base-pairs with adenine and replaces thymine during DNA transcription. Methylation of uracil produces thymine.[10] In DNA, the evolutionary substitution of thymine for uracil may have increased DNA stability and improved the efficiency of DNA replication (discussed below). Uracil pairs with adenine through hydrogen bonding. When base pairing with adenine, uracil acts as both a hydrogen bond acceptor and a hydrogen bond donor. In RNA, uracil binds with a ribose sugar to form the ribonucleosideuridine. When a phosphate attaches to uridine, uridine 5'-monophosphate is produced.[6]
Uracil undergoes amide-imidic acid tautomeric shifts because any nuclear instability the molecule may have from the lack of formal aromaticity is compensated by the cyclic-amidic stability.[5] The amide tautomer is referred to as the lactamstructure, while the imidic acid tautomer is referred to as the lactim structure. These tautomeric forms are predominant at pH 7. The lactam structure is the most common form of uracil.
- → H3NCH2CH2COO− + NH4+ + CO2
Oxidative degradation of uracil produces urea and maleic acid in the presence of H2O2 and Fe2+ or in the presence of diatomic oxygenand Fe2+.
Uracil is a weak acid. The first site of ionization of uracil is not known.[11] The negative charge is placed on the oxygen anion and produces a pKa of less than or equal to 12. The basic pKa = -3.4, while the acidic pKa = 9.389. In the gas phase, uracil has 4 sites that are more acidic than water.[12] a